Problematic Ethics Statements on "Chinese Medicine"
Problematic ethics statements are found in multiple articles on "Chinese Medicine", a Springer Nature title. Here are some examples:
10.1186/s13020-026-01337-9
In-consistent ethical approval numbers are disclosed at two separate parts of this article. First time, it is disclosed on the "Materials and methods" section, and it reads "All animal experimental procedures were strictly conducted in accordance with protocols approved by the Animal Ethics Committee of Shanxi University of Chinese Medicine (Ethics Approval No.: 2021DW256)". Second time, it is disclosed on the "Ethics declarations" section, and it reads "All mouse protocols were approved by the Animal Welfare Committee of Shanxi University of Chinese Medicine (no. 2019DW233)".
We also note that the approval number 2019DW233 is also cited by another article [1], sharing the same first author with this article. However, in article [1], authors state that the animals were raised at the First Affiliated Hospital of Zhengzhou University, while the ethical approval was granted by the committee at the Shanxi University of Chinese Medicine. This is also very weird.
10.1186/s13020-025-01296-7
The authors state on the "Ethics declarations" section that "All animal experiments were performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Experimental Laboratory Animal Committee of the Institute of Medicinal Plant Development, Peking Union Medical College (approval number: SLXD-20180706)". However, no author from Peking Union Medical College is listed on this article. The authors are ether from Chengdu University of Traditional Chinese Medicine, or Capital Medical University. On the other hand, no experiment is claimed to be conducted at Peking Union Medical College.
10.1186/s13020-025-01317-5
In-consistent ethical approval numbers are disclosed at two separate parts of this article. First time, it is disclosed on the "Materials and methods" section, and it reads "The experiments were approved by the Animal Ethics Committee of NJUCM (Application Number: 202203A069) on 8th Mar, 2022". Second time, it is disclosed on the "Ethics declarations" section, and it reads "The animal study protocol was approved by the Animal Care and Use Committee (ACUC) of [Nanjing University of Traditional Chinese Medicine], protocol number [ACU240404]".
We also note that the protocol ACU240404 was also cited on a preprint [2], which shares common authors with this article. The preprint [2] cites another ethical approval, it reads "The experiments were approved by the Animal Ethics Committee of NJUCM (Application Number: 202403A069) on 8th Mar, 2024". The authors claim the these two approvals were both granted on March 8th, but in different year (2022 and 2024), and the approval numbers share the same last six digits with a common segment (03A069). These suggest that the approval numbers may be false.
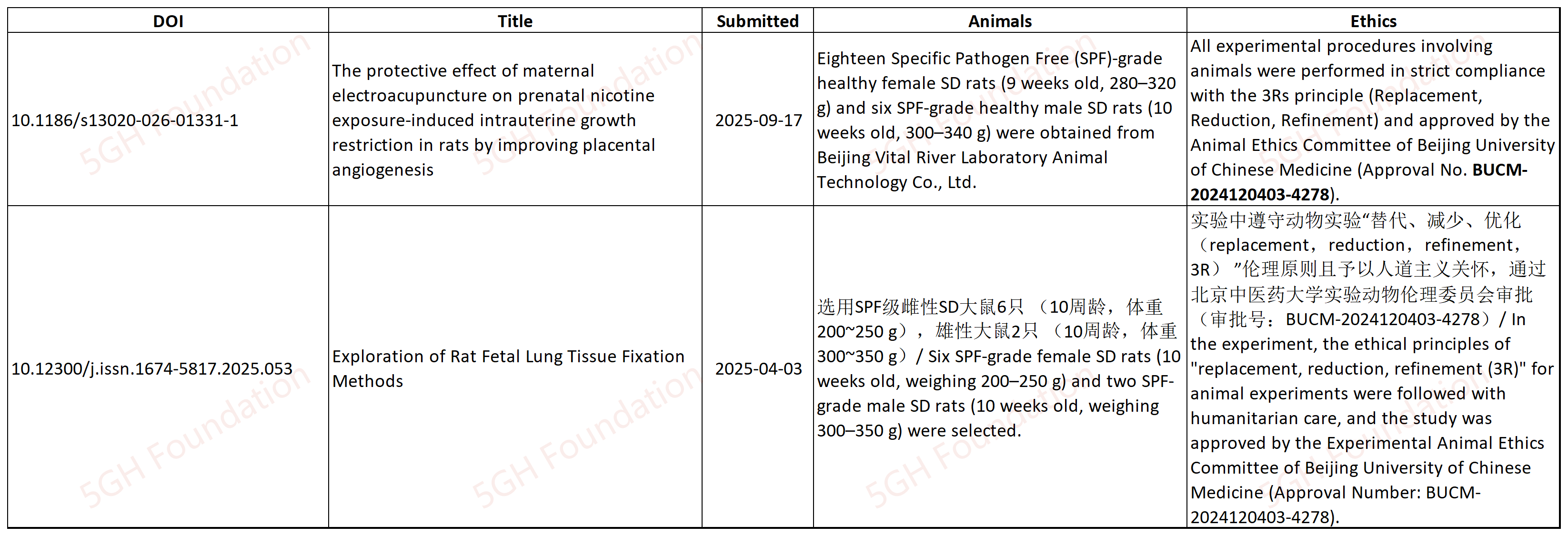
10.1186/s13020-026-01331-1
This articles shares the same ethical approval with other article [3] published on a Chinese journal, describing different experiments.
10.1186/s13020-025-01295-8
In this article, authors presented their research of developing a a large language model (LLM) assistant (GastroTCM) for gastroenterology, which involved fine-tuning a Llama3-8B model and augmenting it with a Retrieval-Augmented Generation (RAG) and an agent framework. Medical records were used in this research, and the authors claimed that the ethical approval was granted on April 13th, 2020. This is questionable. For one hand, such kinds of researches usually emerged following the development of large language models, such as ChatGPT. It is unusual to have obtained ethical approval prior to ChatGPT’s release. For the other hand, whether the medical records collected before the are when general public aware of the LLMs' capabilities can be legitimately used to fine-tune an LLM remains controversial.
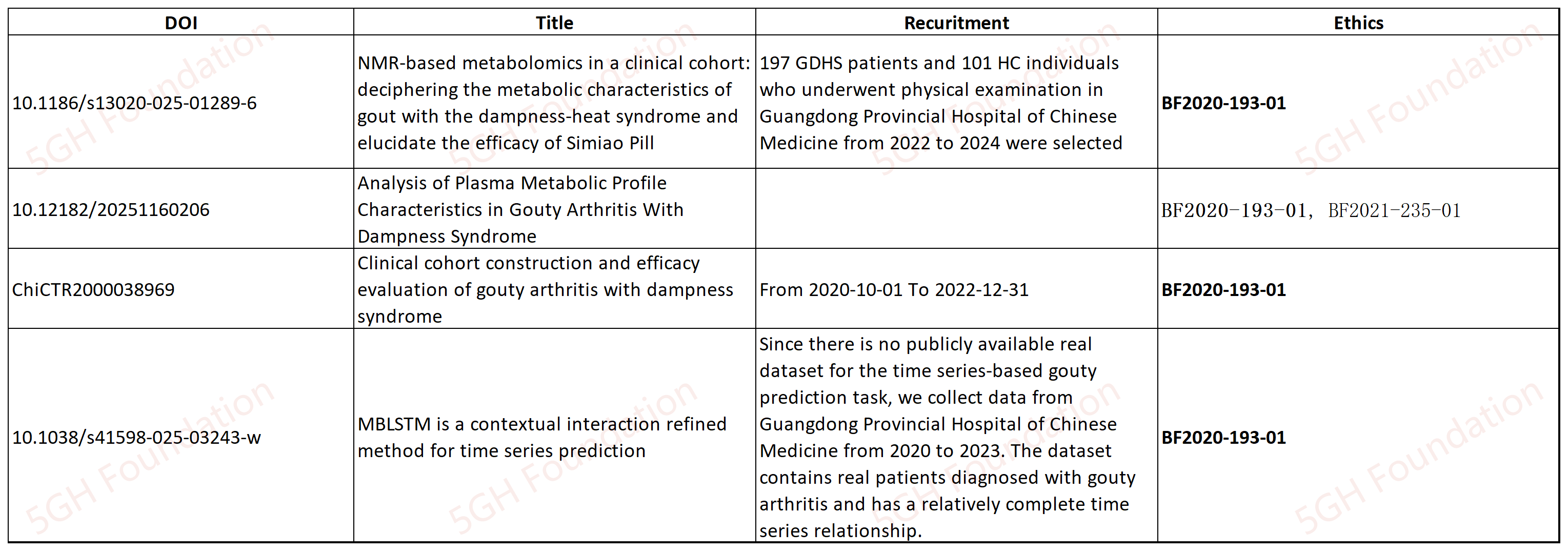
10.1186/s13020-025-01289-6
This article cites the ethical approval BF2020-193-01, which was granted for the registered trial ChiCTR2000038969. According to the document published on the ChiCTR website, the recruitment for the ChiCTR2000038969 trial began on October 1st, 2020, and concluded on December 31st, 2022. However, this article claims that the participants involved in the study were recruited between 2022 and 2024.
We also note that another article [4] also cites the ethical approval number BF2020-193-01. However, the article [4] states the participants were recruited between 2020 and 2023. These inconsistencies suggest that the ethics statement on this article as well as article [4, 5] may be inaccurate or misleading, raising concerns about potential violations of China’s ethics management regulations by the author group.
10.1186/s13020-025-01322-8
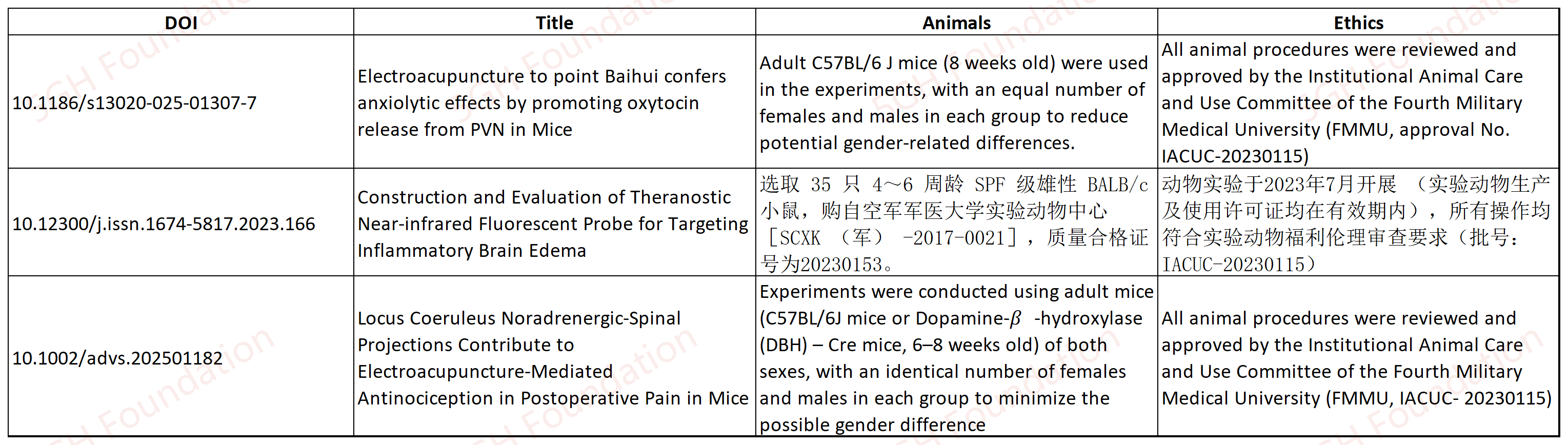
This article cites an ethical approval from Animal Experimental Ethics Committee of Shanghai Second People’s Hospital (2025-0027), suggesting the approval was granted no earlier than January 1st, 2025. The authors should brought the animals after their protocol was granted by the ethics committee, and the treatments to the animal lasted for 10 weeks, after that, the authors analyzed the tissues obtained from the animal, and interpreted the data, prepared the manuscript. This process usually takes long time over a year. However, the manuscript was submitted to the journal on September 2nd, 2025, less than nine months after the approval was granted. This unusual fast process raises concern that the author may started their experiment before they obtain the ethical approval, or that the ethical approval claimed in this article may be false.10.1186/s13020-025-01307-7
This article shares the identical ethical approval number (IACUC-20230115) with two other articles [6, 7], both of which describe distinct animal experiments, raising concerns that the experiments outlined in at least two of these works were not formally approved by the ethics committee, thereby potentially violating China’s ethics management regulations.
10.1186/s13020-025-01321-9
This article lists an ethical approval number of KY20231295. Notably, this approval was issued by the same ethics committee as referenced in article [8], yet the approval numbers differ in format, KY20231295 in this work versus IACUC-20230115 in Article [8]. These discrepancies raise concerns that the ethical approval cited herein may have been granted for securing funding rather than for the specific experiment described in this study.
Reference
[2] 10.21203/rs.3.rs-7844488/v1
[3] 10.12300/j.issn.1674-5817.2025.053
[4] 10.1038/s41598-025-03243-w